HFB301001: OX40 Agonist
Phase I, Recruiting
Targeting OX40 with a next generation agonist antibody for cancer immunotherapy
OX40 is a TNF superfamily receptor expressed on activated CD4+ and CD8+ T cells and regulatory T cells. Several 1st generation OX40 agonists have shown modest clinical efficacy in cancer patients. Multiple factors, including OX40 target desensitization and competition with OX40 ligand, have been associated with the limited efficacy of these antibodies.
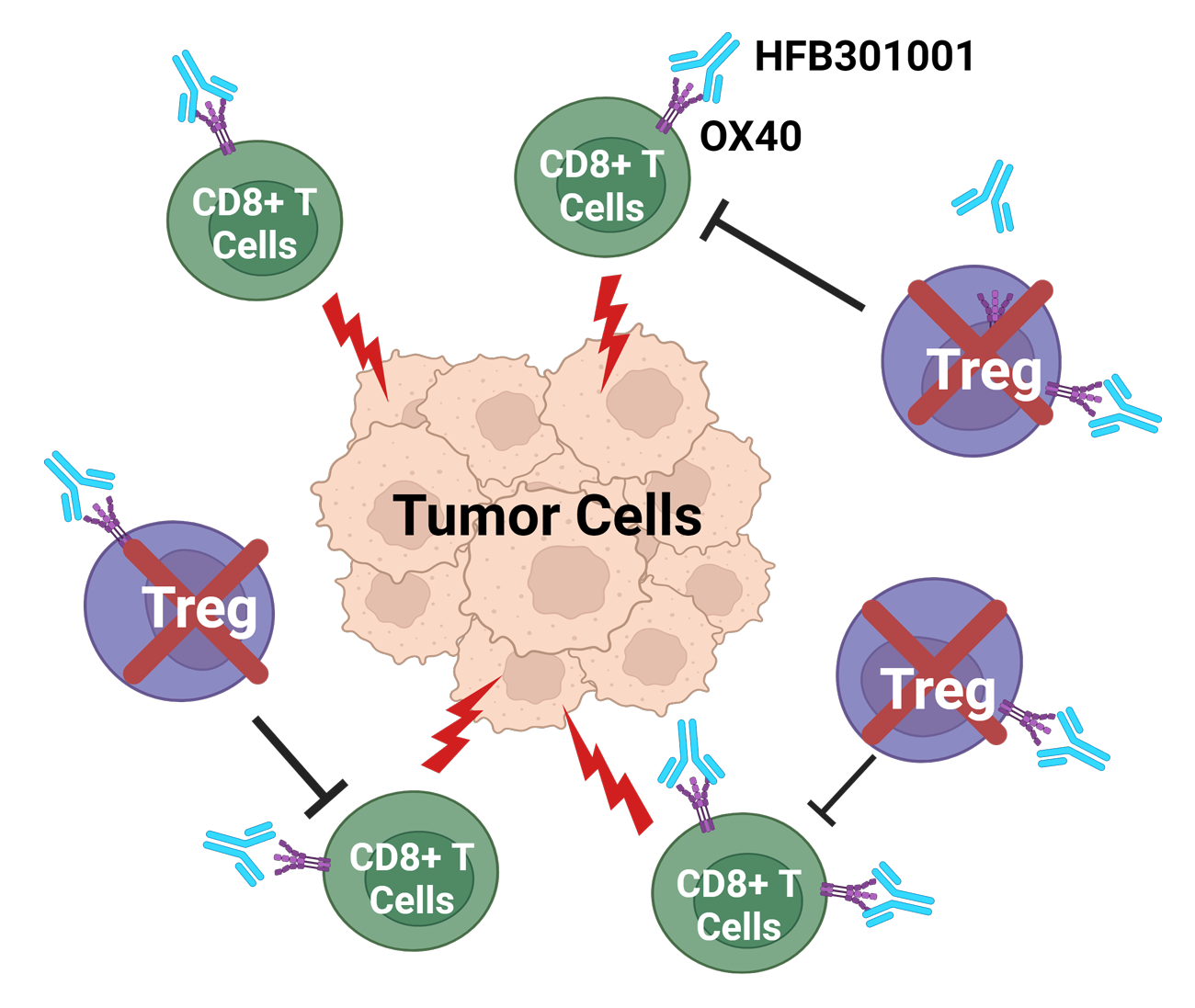
The clinical efficacy of prior OX40 agonists evaluated in cancer patients has thus far shown limited effects. Several functional limitations of these ‘first generation’ OX40 agonists have been associated with their limited clinical efficacy. OX40 agonists can be designed differently to potentially address the limitations of these early attempts at targeting OX40 agonism.
Our DIS® Approach
DIS® has been utilized to design an antibody that addresses the limitations of 1st generation OX40 agonists and to identify the patients who can benefit the most from treatment. Ongoing analysis of clinical trial samples to identify features unique to patients responding to treatment will enable patient selection in future biomarker-guided trials.
Drug Info
HFB301001 is a novel fully human IgG1 class OX40 agonist antibody with an optimized pharmacological profile. In contrast to other anti-OX40 antibodies, the agonistic activity of HFB301001 is further enhanced in the presence of the endogenous ligand (OX40L) and does not result in the reduced expression of OX40 on T cells. HFB301001 demonstrated superior anti-tumor activity in a mouse model compared to 1st generation OX40 agonists. HFB301001 has shown an excellent safety profile, demonstrated proof of mechanism and shown promising monotherapy activity in early clinical studies.
Clinical Trial Info
The ongoing first-in-human dose escalation study (NCT05229601) evaluates HFB301001 as a monotherapy in patients with DIS®-selected advanced cancers. Identification of biomarkers predictive of response is ongoing.
