HFB200301: TNFR2 Agonist
Phase I, Recruiting
Stimulating TNFR2 signaling in effector T and NK cells to promote anti-tumor immunity
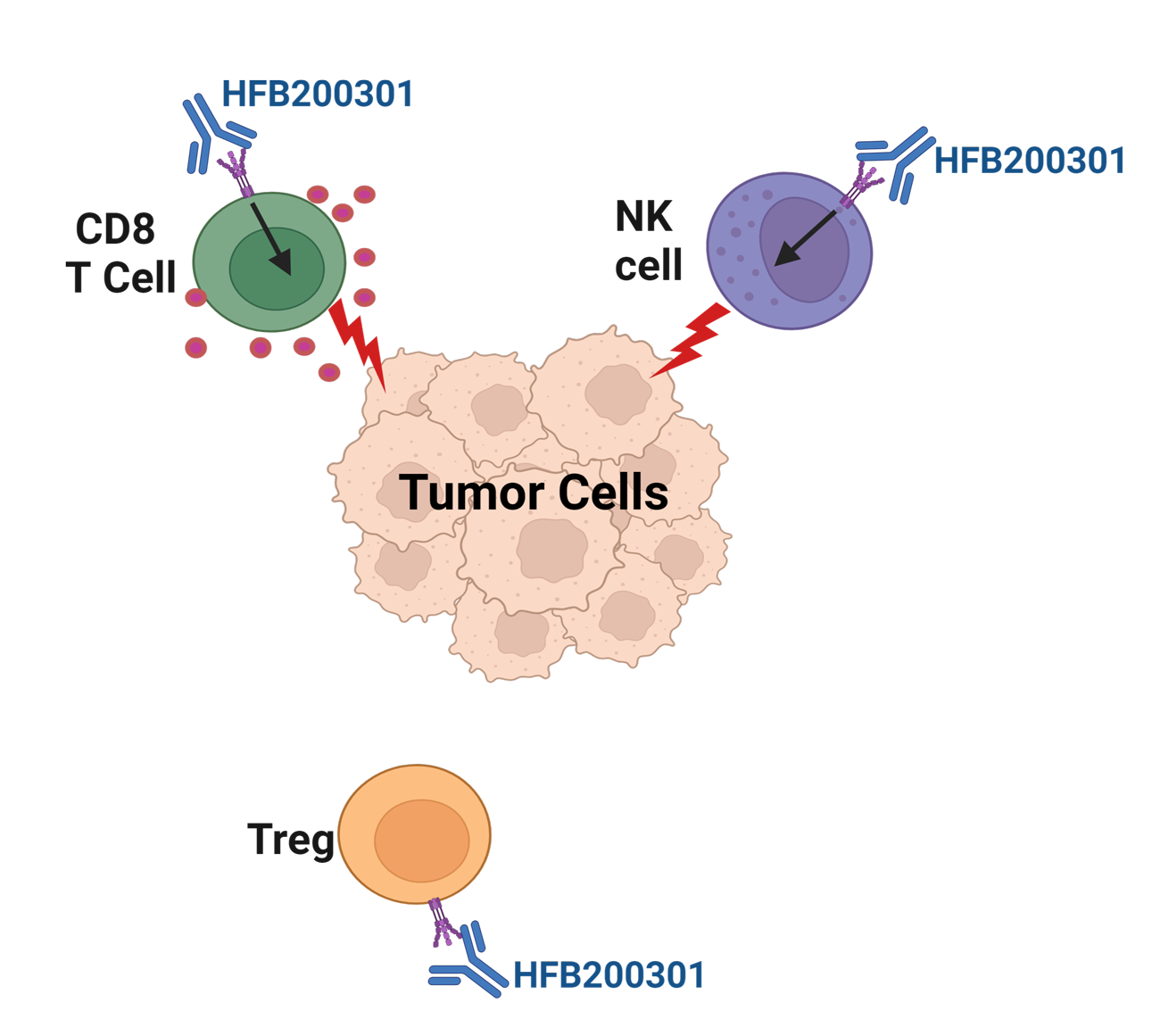
TNFR2 (tumor necrosis factor receptor 2) expression is highly regulated and restricted to immune cells, including CD4+, CD8+ T cells, NK and myeloid cells. TNFR2 is primarily activated by its ligand, transmembrane TNFα, to drive immune cell activation, proliferation, and survival. Based on TNFR2’s ability to augment the antitumor responses of effector CD8+ T cells and NK cells, TNFR2 agonism represents a rational therapeutic mechanism for cancer immunotherapy.
Our DIS® Approach
Anti-PD-1 immunotherapy has demonstrated efficacy across many cancers; however, many patients develop resistance to treatment, revealing a large unmet need for new immune modulators that can overcome this resistance. HiFiBiO’s DIS®-guided single-cell analysis has shown high TNFR2 expression on cytotoxic T cells in tumors not responding to anti-PD-1 treatment. Stimulation of TNFR2 on these cells with an agonist antibody may enhance anti-tumor activity and overcome resistance to PD-1 blockade.
Drug Info
HFB200301 is a first-in-class immune cell agonist with an excellent safety profile and promising clinical activity in monotherapy and in combination with anti-PD-1. Through binding to a unique epitope, it stimulates TNFR2 inducing NF-κB signaling and activation and proliferation of CD4 + and CD8 + T cells and NK cells. This immune cell expansion has been shown to reduce tumor burden in mouse models. Preclinical studies show that HFB200301 has an even greater effect when combined with PD-1 blockade. This, together with its favorable pharmacokinetic and safety profiles, supports HFB200301 clinical development in solid tumors.
Clinical Trial Info
The first-in-human dose escalation and expansion study (NCT05238883) is actively enrolling patients with DIS®-selected advanced cancers for treatment with HFB200301 monotherapy or combination with anti-PD-1 tislelizumab. Identification of biomarkers predictive of response is ongoing.
